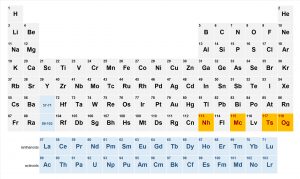
The periodic table is getting four new names added to it. And it looks like you’ll need to throw out your now outdated shower curtain.
The four proposed element names taking the final spots on the 7th row of the table are: nihonium (Nh, element 113), moscovium (Mc, element 115), tennessine (Ts, element 117), and oganesson (Og, element 118).
The elements themselves were discovered years ago but the International Union of Pure and Applied Chemistry (IUPAC) announced the proposed names on June 8th. In keeping with tradition, the IUPAC let the discoverers propose the name of the new elements within a set of guidelines. The element can be named after: (1) a mythological concept or character, (2) a mineral or similar substance, (3) a place or geographical region, (4) a property of the element, or (5) a scientist.
And yes, as it states in #1, you can name an element after a mythological figure. That’s how we got thorium – named after the Norse god of thunder.
Of the four newest elements, three were named after the places in which they were discovered while the fourth – oganesson – was named after a living scientist.
Nihonium is named after Nihon, which translates into ‘land of the rising sun’ and is how you pronounce Japan’s name in Japanese. It was discovered at the RIKEN Nishina Center for Accelerator-Based Science and marks the very first element to be discovered in an Asian country.
As you might have guessed, moscovium is named after the Moscow region in which it was discovered while tennessine gives credit to Tennessee.
Oganesson honors Russian physicist Yuri Oganessian, a pioneer in the discovery of superheavy elements. This is only the second time that an element is being named after a living person. Seaborgium was the first – named after American Nobel Prize winning chemist, Glenn Seaborg, in 1997.
At that time, the idea of naming an element after a living scientist caused a huge controversy and seaborgium wasn’t recognized for several years while a prolonged battle between different factions of the IUPAC played out. It just goes to show how heated this process can get. Just look at the hundred-years war over the naming of niobium.
So what do these new discoveries mean for science? Well, at this point in the periodic table, elements are getting so heavy, they’re incredibly unstable. All are created in laboratory conditions and most live for less than a second before breaking apart into smaller elements. But what scientists are looking for is an elusive element called the island of stability. The idea was put forth by Glenn Seaborg and theorizes that there should be an unusually stable element somewhere among these superheavy giants. If we can find it, we could potentially use it to build things we haven’t even imagined yet. So we now know none of the elements up to 118 are that island of stability. But scientists are continuing to march forward and are looking at ways to unlock the 8th row.
And to be clear, the new names for the last four elements are not finalized yet. The public has until November 8th to raise objections. But don’t worry, you probably won’t see any kind of drama on the level of Boaty McBoatface for these four elements. They’re expected to sail smoothly to an approval come November.